Critical Limb Ischemia (CLI) - LSTA12 (HONEDRA® in Japan)
What is Critical Limb Ischemia (CLI) and Buerger’s Disease?
Also known as Chronic Limb-Threatening Ischemia (CLTI)
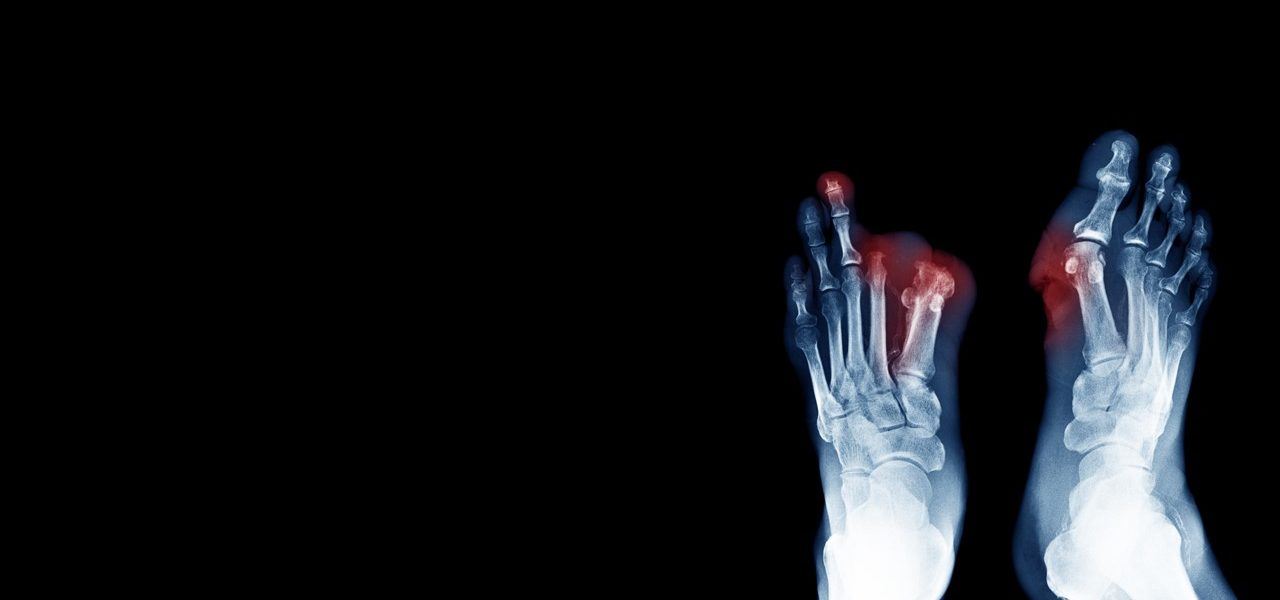
Critical limb ischemia (CLI) is a severe obstruction of the arteries which markedly reduces blood flow to the extremities (hands, feet, and legs). CLI is the advanced form of peripheral arterial disease (PAD) caused by atherosclerosis, the hardening and narrowing of the arteries over time due to the buildup of fatty deposits called plaque.
Critical Limb Ischemia is a chronic condition that results in severe pain in the hands, feet or toes, even while resting. Complications can include ulcers and wounds on the extremities that won’t heal due to the lack of circulating blood (carrying oxygen) essential to our body’s healing process. Left untreated, the complications of CLI can result in amputation of the affected limb.
What is Buerger’s Disease?
Buerger’s disease is a rare disease of the arteries and veins in the arms and legs. In Buerger’s disease – also called thromboangiitis obliterans – your blood vessels become inflamed, swell and can become blocked with blood clots. This eventually damages or destroys skin tissues and may lead to infection and gangrene (the death or decay of body tissues).(1). In some cases, amputation may be required. (2). The largest risk factor for Buerger’s disease is tobacco abuse.
Critical Limb Ischemia represents a large unmet medical need
In Japan, there are approximately 300,000 patients with CLI, of whom approximately 51,000 are not candidates for revascularization, making them the addressable population for CLBS12. The addressable population is approximately 560,000 patients in the EU and 300,000 in the U.S.
Buerger’s disease is extremely rare in the U.S. and Europe, but more common in Asia and the Middle East. The incidence in the U.S. has been estimated to 12.6-20 per 100,000 people in the general population. Buerger’s disease occurs with greater frequency in countries that have heavy tobacco use. (3)
Our Approach: HONEDRA® for the treatment of critical limb ischemia
HONEDRA®, an experimental regenerative medicine, is the Company’s SAKIGAKE-designated product candidate for the treatment of Chronic Limb Ischemia and Buerger’s disease in Japan. Its goal is to prevent the serious adverse consequences of CLI and Buerger’s disease by improving blood flow in the affected limb.
Investigational Clinical Trial of HONEDRA®
Lisata’s randomized and open-label, registration-eligible study of HONEDRA® in Japan for the treatment of CLI and Buerger’s disease, diseases with limited therapeutic options, has shown promising results to date. The initial clinical responses are consistent with a positive therapeutic effect and safety profile and are consistent with previously published clinical trials of CD34+ cell therapy in Japan and elsewhere. The Company is conducting ongoing discussions with the Japanese Pharmaceuticals and Medical Devices Agency (PMDA) as to what needs to be considered in preparation for the formal consultation meetings which precede the Japanese new drug application. Simultaneously, the Company is focusing its efforts on securing a Japanese partner to complete the remaining steps to produce registration in Japan.
| Pre Clinical | Phase 1 | Phase 2 | Phase 3 |
|---|---|---|---|
|
Phase 2
|
|
Phase 2
|
|
| Indication: | Critical Limb Ischemia and Buerger’s Disease |
| Partner/Sponsor: | Lisata | Location: Japan |
| Next Development Milestone: | PMDA consultation underway |
